Current Focus
Ordered by current priority and time commitment
Wearable Emergency Medical Device
Portable, wearable emergency intervention device designed to improve outcomes in time-critical situations. Development is progressing with provisional patent protection in place.
Public details are intentionally limited. Technical documentation available under NDA for qualified parties.
Alzheimer's Disease Drug Development
Development of an Asparagine Endopeptidase (AEP) inhibitor for Alzheimer's disease. Currently in lead optimization stage with mechanistic and translational work supporting clinical development pathway.
Dog Food Supplements
Animal health and nutrition business, maintained with limited day-to-day involvement. Established operational framework supports ongoing activities.
Featured Project: Wearable Emergency Medical Device
Out-of-hospital cardiac arrest remains a leading cause of sudden death worldwide. Survival depends heavily on the speed, consistency, and quality of the initial intervention. Existing automated systems can be effective, but their size, weight, and setup requirements can limit real-world usability in constrained or mobile environments.
Designed to achieve
- Consistent, guideline-aligned emergency intervention
- Wearable, highly portable form factor
- Rapid deployment in time-sensitive settings
- Reduced reliance on manual execution under fatigue or stress
Intended contexts
- Emergency medical services
- Defense and tactical settings
- Hospital and critical-care environments
- High-risk patient monitoring
Competitive Positioning
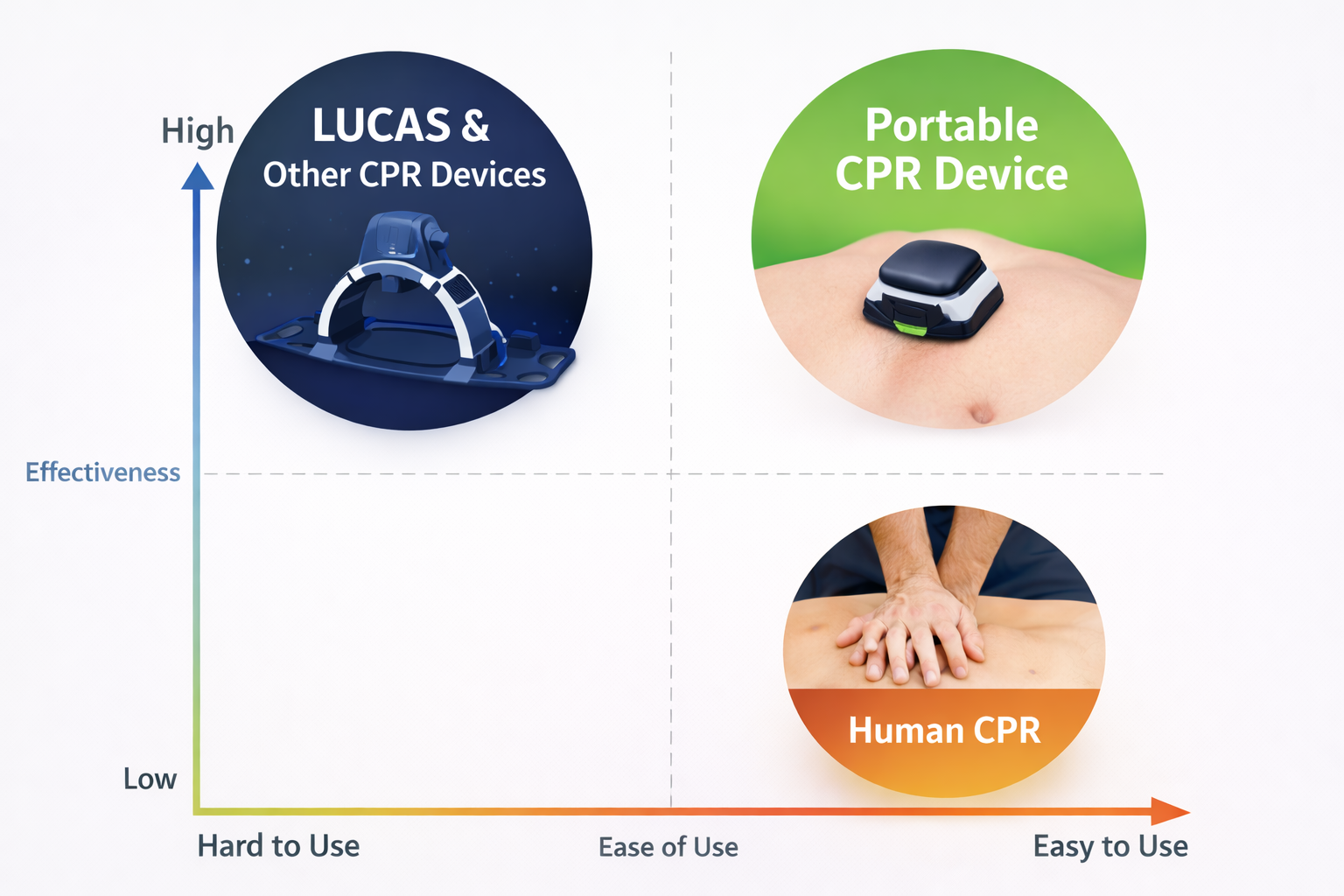
IP Note: A USPTO provisional patent application has been filed. Public information intentionally remains high-level. Technical documentation and detailed materials can be shared under NDA.